Product description
Hydrogen fuel cell system developed by H2SYS is the result of 15 years of expertise in the field of hydrogen energy.
To operate a fuel cell, the stack must be integrated into a system which is called Balance of Plant. It includes all the auxiliaries such as cooling, air intake, solenoid valves, safeties, communication board, T°C and pressure sensors, electronics, fuses, cables …
Aircell fuel cell integrated a Balance of Plant patented by H2SYS with specific components that meet CE standards for both stationary and embedded application. System is ready to use and easy to connect.
Air Cooled System – ACS range – meets various application. It can be used both for light mobility application (range extender for machine or vehicle), inside small genset (battery pack and fuel cell with inverter), or for stationary application (use with electrolyzer).
Universities can also use the Aircell product for research projects.
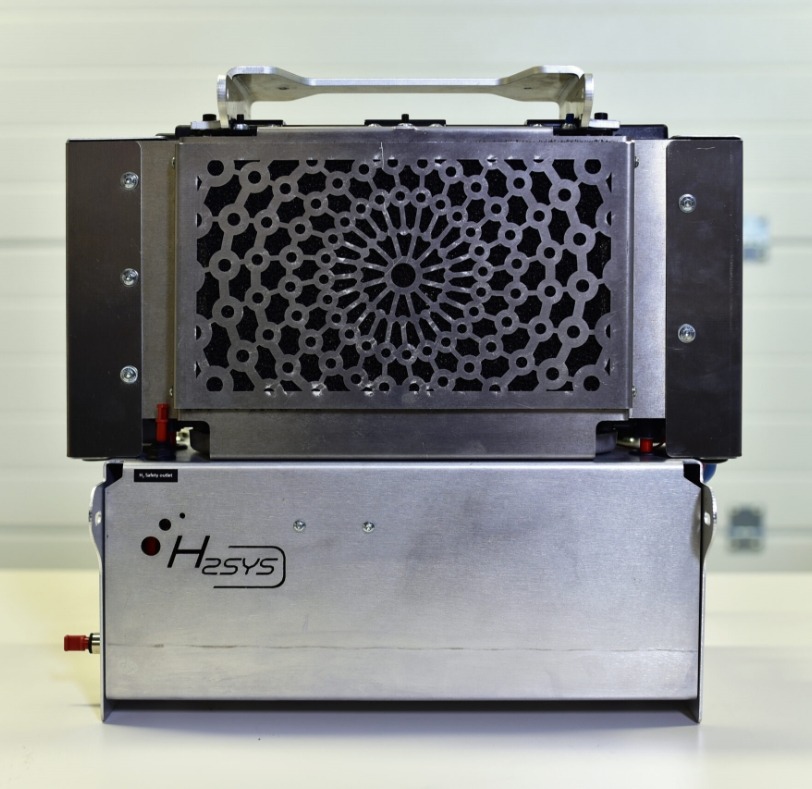
Product features
Communication through Modbus TCP/IP
Gateway that converts Canbus communication frames to ModBUS communication frames.



