What is hydrogen ?
Hydrogen is the most common element in the universe. That is the main component of gaseous planets and stars, but it is rarely found in free state on Earth.
Hydrogen bonds with other elements. With oxygen it forms water (H2O) and combined with carbon it produces hydrocarbon (CH4, C2H6, …).
A hydrogen atom is mostly combined with another hydrogen atom to create the “dihydrogen molecule” (H2). This one can be found in a gas state or in a liquid state – represented by the acronym LH2 – by cooling the gas at -252.87°C.

Where can we find hydrogen?
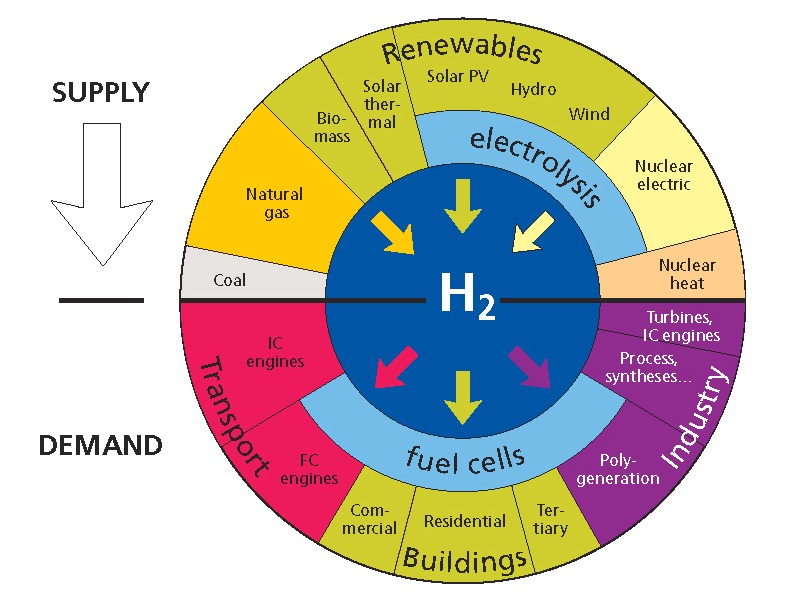
Hydrogen is a natural element that can be extracted from other molecules such as water. For example by electrolysis of water using renewable energy sources, or by reforming other energy sources such as natural gas or oil.
How is hydrogen produced?
The world of science debates on potential natural hydrogen sources in Russia and others places. Nevertheless these sources are not industrialized and two main methods are used to industrially produce hydrogen:
Benefits of using hydrogen
The H2 molecule of hydrogen (commonly known as hydrogen) consists of two hydrogen atoms. It presents an interesting energy density (per unit fuel mass) that can be exploited through technological and scientific research
Progress expected for the democratization of hydrogen
Hydrogen technology has largely been developed during the last few years thanks to scientific and technological achievements. Nevertheless, there is still room for innovation and a lot of research efforts have been committed to overcome various barriers:
The widespread use of hydrogen requires working proactively on the hydrogen sector to improve technology, to increase efficiency and to embed hydrogen into existing technologies to make the most of its advantages
The place of hydrogen in the energy transition
Hydrogen was at the heart of the debate at COP 21 held in Paris in December 2015. Hydrogen will play an important role in the energy transition in addition to renewable energy.
Renewables are intermittent and may produce more energy by the time that the network can absorb or even does not produce enough energy to meet network demands.
This phenomenon is observed with photovoltaic solar panels that produce an energy surplus between 10h and 16h but do not cover a home’s needs in the morning or in the evening.
Hydrogen allows, in particular, to store a surplus of energy compared to intermittent energies as it overcomes the constraints of decentralized production and finally its use can significantly reduce emissions of greenhouse gases.
Hydrogen thus has a relevant role in the energy transition, though its industrial operation still requires a lot of investment.

How to use hydrogen ?
As an energy carrier, hydrogen can be converted to electricity, heat or kinetic energy and can be used:
For stationary applications, through the production of electricity and/or heat in buildings (principle of combined heat and power production)
For industrial applications by using hydrogen as a chemical compound
For mobile applications by using hydrogen as a driving force
The conversion of hydrogen to electricity requires an energy converter: the hydrogen fuel cell (or fuel cell)